Sample Transport
Culture ☆ Chromogenic Result

Categories
InTray® COLOREX™ KPC
Quick Links: Specifications Detection Incubation Storage Whitepapers
Chromogenic differentiated result, in your clinic.
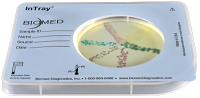
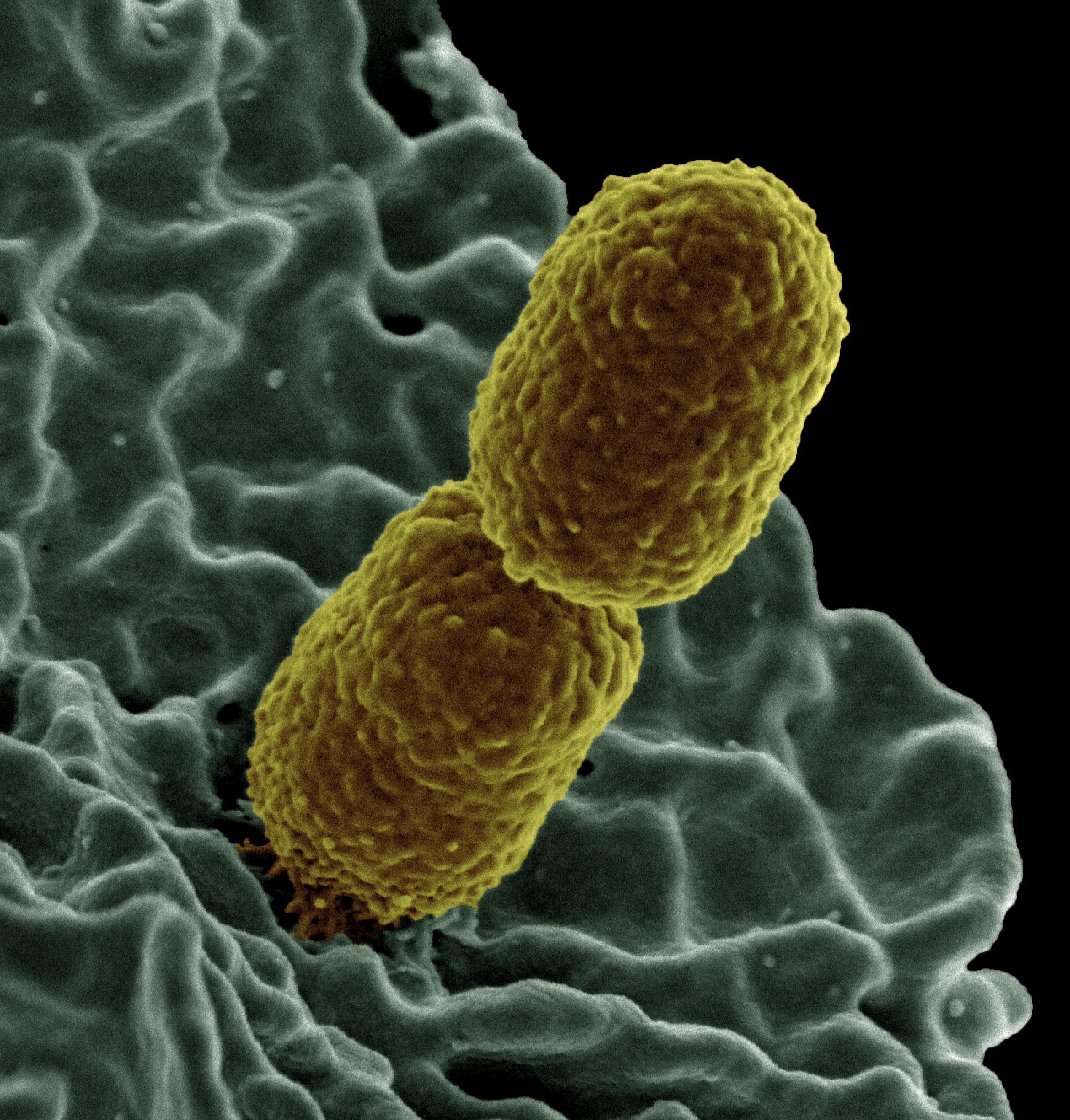
COLOREX KPC is a selective and differential chromogenic medium containing a carbapenem selective agent intended for use in obtaining a pure culture of gram-negative bacteria expressing reduced susceptibility to carbapenem-class antibiotics. The test can be performed with samples composed of mixed populations of bacteria, e.g., stool, biological fluids, surface streaks, etc.
Specifications
| Product Principle |
|
|---|---|
| Simple Convenience |
|
| Accurate Detection | |
| Time to Result |
|
| Storage | |
| Specimen |
|
Features and Benefits
SHELF-LIFE AND DURABILITY
- Designed for extended 6 month shelf life
- Durable and stackable for incubation
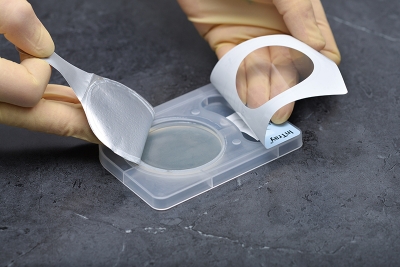
- No re-plate or wet mount
- Once inoculated, no other culture preparation is required... saving time and cost.
- Designed for specimen collection/inoculation at point-of-care, a fully enclosed system for transport, incubation, result and microscopic observation.
- The sealed prepared media device is selectively formulated for accurate detection and identification of pathogens and non-pathogens
- Visual chromogenic differentiation and/or selective production of distinctive colony growth with typical identifiable morphology
KEY FEATURES
- Fully enclosed cassette with air filtration system
- Nutritive culture colony growth medium, formulated for selectivity and specificity
- Anaerobic or aerobic system available (on select products)
YOU'LL IMMEDIATELY EXPERIENCE A REDUCTION IN:
- Time-to-result
- Cost of laboratory materials
- Medical waste
- Risk of exposure or contamination
Articles and Whitepapers
Laboratory and Clinical Evaluation of Screen Agar Plates for Detection of Carbapenem-Resistant Enterobacteriaceae from Surveillance Rectal Swabs
Adler et al, Laboratory and Clinical Evaluation of Screening... JOCM 2011 Jun; 49(6)
Clinical Microbiology Costs for Methods of Active Surveillance for Klebsiella pneumoniae Carbapenemase — Producing Enterobacteriaceae
Mathers, MD et al, Microbiology Cost of CPE Screening, Infection Control and Hospital Epidemiology 2014, Vol 35 No 4
Evaluation of CHROMagar KPC for Rapid Detection of Carbapenem-Resistant Enterobacteriaceae
Samra Z, Bahar J., Marad-Shapiro L., J Clin Microbiol. 2008 Sept; 46(9)3110-3111
Outbreak of ST395 KPC-Producing Klebsiella pneumoniae in a Neonatal Intensive Care Unit
Stein et al, Outbreak of ST395 KPC...,IC&HE 2018 Vol 39, No 4
Identification of Klebsiella pneumoniae Carbapenemase-producing Klebsiella oxytoca in Clinical Isolates in Tehran Hospitals
Validi et al, ID of KPC-producing Klebsilla oxytoca...Osong Public Health Res Perspect 2016 Vol 7 No 5
Regulatory
CE Marked
FDA Registered in USA - For Research Use Only
COLOREX KPC is not intended for use in the identification of colonization with carbapenem-resistant bacteria to aid in the prevention and control of carbapenem-resistant bacteria in healthcare settings.
COLOREX KPC is not intended to diagnose infections with carbapenem-resistant bacteria, guide or monitor treatment for infections, or provide susceptibility results to carbapenem. Sub-culture is necessary for bacterial identification and susceptibility testing.