InTray® COLOREX™ MRSA
Quick Links: Specifications Detection Incubation Storage Whitepapers
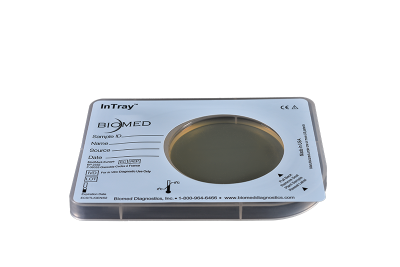
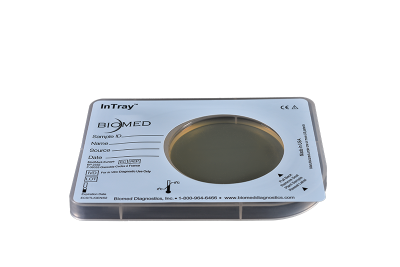
Chromogenic differentiated results for surveillance and environmental testing.
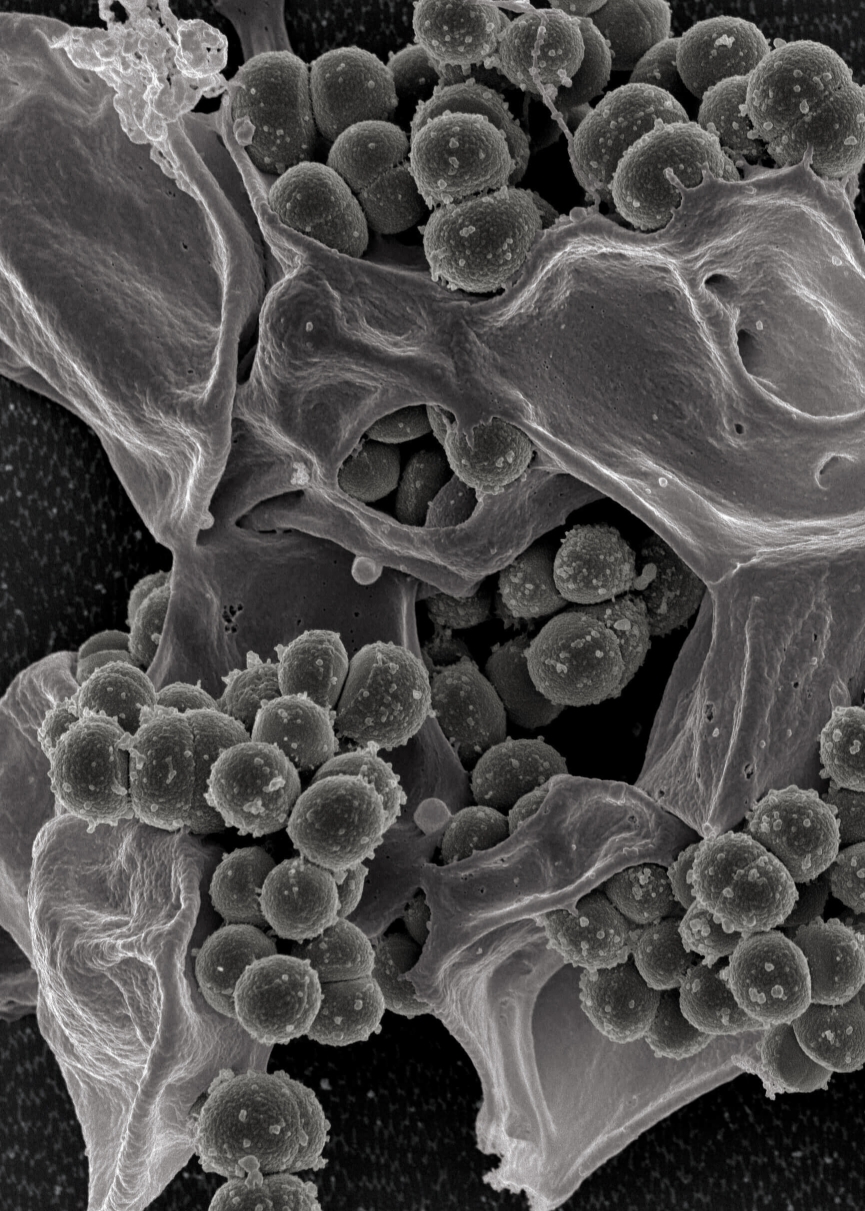
Over recent years, the occurrence of hospital infections caused by methicillin resistant Staphylococcus aureus (MRSA) has been increasing steadily, representing around 20 to 55% of the isolates in Europe and in the USA. Leading cause of nosocomial infections, especially in intensive care units, the MRSA sources are either endogenous (the patient) or through cross contamination (environmental or by person to person contact). The major issue with this pathogen is its resistance to a large panel of antibiotics, among them beta-lactam antibiotics, limiting the therapeutic options for clinicians.
InTray COLOREX MRSA provides an easy-to-use, in-house, chromogenic result for surface testing and AMR surveillance.
Specifications
Features and Benefits
SHELF-LIFE AND DURABILITY
- Designed for durability and stackable for incubation, or 3 month storage.
- No re-plate or wet mount
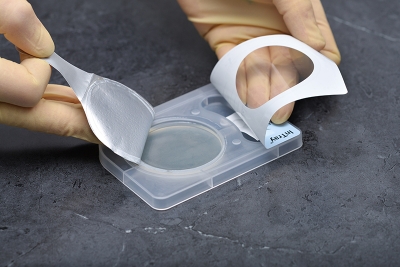
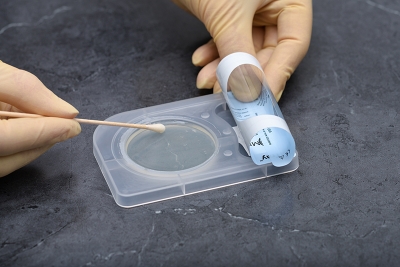
- Once InTray is inoculated, no other culture preparation is required... saving time and cost.
- Designed for specimen collection/inoculation at point-of-care, a fully enclosed system for transport, incubation, result and microscopic observation.
- The sealed prepared media device is selectively formulated for accurate detection and identification of pathogens and non-pathogens
- Visual chromogenic differentiation and/or selective production of distinctive colony growth with typical identifiable morphology
KEY FEATURES
- Fully enclosed cassette with air filtration system
- Nutritive culture colony growth medium, formulated for selectivity and specificity
- Anaerobic or aerobic system available
YOU'LL IMMEDIATELY EXPERIENCE A REDUCTION IN:
- Time-to-result
- Cost of laboratory materials
- Medical waste
- Risk of exposure or contamination
Regulatory
CE Marked
FDA Registered in USA - For Research Use Only
Ordering Information
To order, please:
Articles and Interest
Isaac See, Yi Mu, Valerie Albrecht, Maria Karlsson, Ghinwa Dumyati, Dwight J Hardy, Mackenzie Koeck, Ruth Lynfield, Joelle Nadle, Susan M Ray, William Schaffner, Alexander J Kallen; Trends in incidence of methicillin-resistant Staphylococcus aureus bloodstream infections differ by strain type and healthcare exposure,
United States, 2005–2013, Clinical Infectious Diseases, , ciz158,