New!
Transport Media for SARS-CoV-2 Testing


Categories
VTM-C19 Transit Tube
DISCONTINUED
Quick Links: Specifications Storage Transport Reference
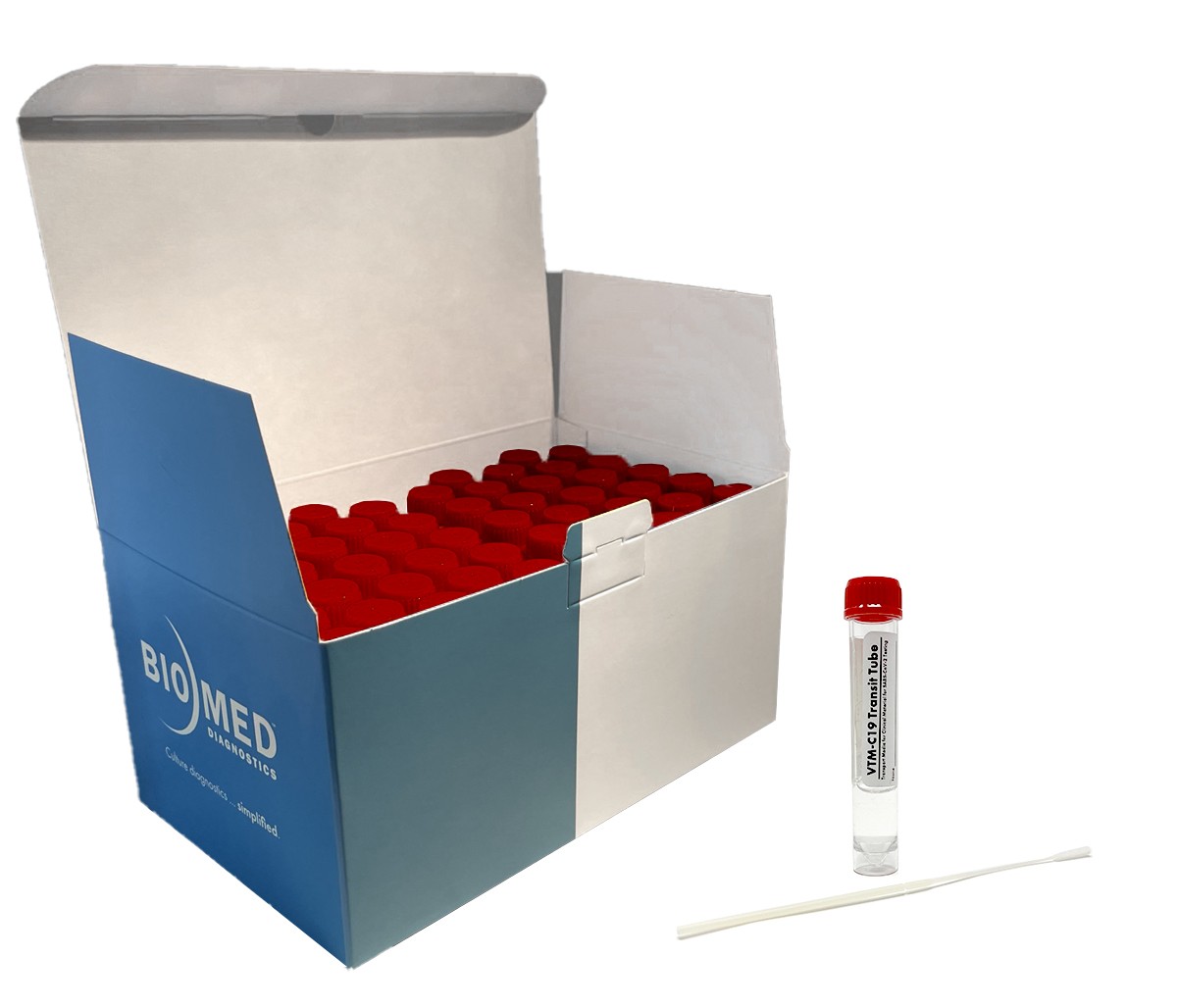
VTM-C19 Transit Tube
A premium transport device for use with nucleic acid testing of SARS-CoV-2 and (USA only) human influenza specimens
Biomed Diagnostics' VTM-C19 Transit Tube is intended for on-site collection and transport to the testing laboratory of human clinical specimens containing SARS-CoV-2 — the virus that causes COVID-19 disease in humans and/or (USA only) influenza A virus. 1–4
Intended to be inoculated with specimens collected with a nasopharyngeal (NP) or oropharyngeal (OP) synthetic fiber swab (not provided), VTM-C19 has been validated to transport SARS-CoV-2 and (USA only) influenza A viral specimen appropriately to the lab for analysis with qRT-PCR.
Specifications
Transportation
The VTM-C19 Transit Tube is designed for safe transport. Inoculated tubes should be transported within 72 hours after inoculation and maintained at 2-8°C. 2
Regulatory
For In Vitro Diagnostic Use.
Not available in all countries; please inquire.
CE IVD marked for use with SARS-CoV-2 testing
The VTM-C19 Transit Tube is available for use in the USA under the FDA guidance “Enforcement Policy for Viral Transport Media During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency” (July 2020). The VTM-C19 Transit Tube has completed the Notification process. The VTM-C19 Transit Tube is available for use in the USA with FDA-cleared molecular influenza tests as described in the FDA guidance “Enforcement Policy for Modifications to FDA-Cleared Molecular Influenza and RSV Tests During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency” (October 2020).4
FAQ
Technical Questions
VTM-C19 transport media is designed for specimen preservation in preparation for analysis with validated qRT-PCR assays to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 disease in humans.
This is CDC-approved media for COVID-19 sample collection and transport. VTM-C19 has been validated for stable transport of SARS-CoV-2 only.
Biomed Diagnostics also offers Saline .85% transport media for a general viral transport – our R&D lab has validated the Saline .85% for SARS-CoV-2, and the Saline is checked throughout the shelf life of the product for sterility by our QC Labs
Reference
- Centers for Disease Control and Prevention (CDC) Laboratory Outreach Communication System SOP post on 3-21-20: cdc.gov/coronavirus/2019-ncov/downloads/Viral-Transport-Medium.pdf
- FDA authorized instructions for use. fda.gov/media/134922/download
- Enforcement Policy for Viral Transport Media During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency: fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-viral-transport-media-during-coronavirus-disease-2019-covid-19-public-health
- Enforcement Policy for Modifications to FDA-Cleared Molecular Influenza and RSV Tests During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency: fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-modifications-fda-cleared-molecular-influenza-and-rsv-tests-during-coronavirus
- CDC preventing transmission of infectious agents in healthcare settings guidelines; cdc.gov/infectioncontrol/guidelines/isolation