Saline Solution (0.85%)
Quick Links: Specifications Storage Performance Reference
For transport of SARS-CoV-2 [COVID-19] specimens
The Saline Solution (0.85%) is an isotonic solution, manufactured in compliance with ISO 13485:2016 standards. It is intended to be inoculated with nasopharyngeal (NP) or oropharyngeal (OP) synthetic fiber swab specimens (Not provided, see IFU for details), to be analyzed in the laboratory with validated qRT-PCR assays. This Saline Solution (0.85%) has been validated for the detection of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 disease in humans.1,2 The use of this device for other viral specimens is the responsibility of the end user including the validation thereof.
Specifications
Performance Characteristics
This test was performed to evaluate the Biomed Saline Solution (0.85%) by detection of heat inactivated cell lysate from SARS-Cov-2 (ATCC® VR-1986HK™) infected cells, after RNA was isolated using the QIAamp® Viral RNA Mini Kit (QIAGEN®). Detection of the isolated SARSCov-2 RNA was by qRT-PCR using New England Biolabs® OneTaq® One-Step RT-PCR kit and run on a Roche® Lightcycler® 96 with EvaGreen® (Biotium®) detection from samples after storage of viral lysates in Saline Solution (0.85%) at 24, 48 and 72 hours at room temperature (25°C). Synthetic RNA standard (BEI Resources) at 2.9 x 108 was serially (10-fold) diluted for the standard (STD) in the amplification reaction.
The assay results demonstrate consistent amplification and Cq quantification of the nCov-2 N gene (using the N1 CDC primer) in the solution after incubations. In addition, excellent dilution linearity is demonstrated across all samples and replicates (See Figure 1) indicating consistency in the performance of the solution Saline Solution (0.85%) replicate samples and incubation time. This data indicates that the Biomed ISO 13845 manufactured Saline Solution (0.85%) does not negatively interfere with the qRT-PCR detection of Sars-Cov-2 viral nucleic acid materials after incubation at room temperature 25°C for 72 hours. A delay in amplification signal was observed after 168-hour (7 days) incubation at 25°C, probably due to specimen degradation. However, incubation at 4°C for 168 hours (7 days) did not negatively interfere with the qRT-PCR performance. In conclusion, the result indicates that the Saline Solution (0.85%) is compatible with the designated viral specimen inoculation, nucleic acid extraction and qRT–PCR assay when used as described herein.
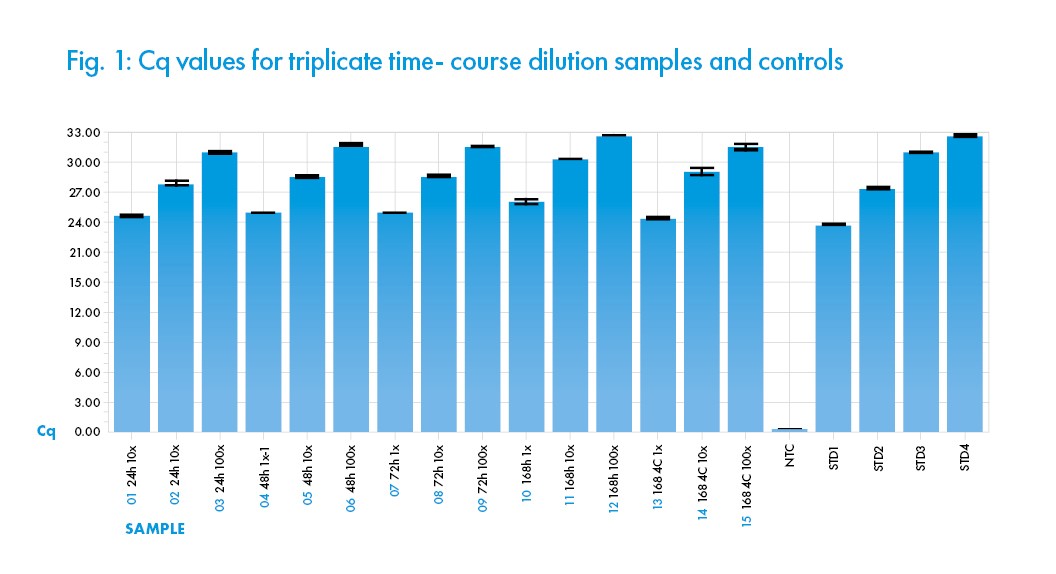
All samples shown are means from triplicate assays, with error bars indicating the arithmetic error for Cq shown. The performance of the qRT-PCR reaction assessed with standard curves indicated R2 of 0.98, Efficiency of 114%, and Slope of -3.033. All the values fall within acceptable quantitative PCR ranges.
Regulatory
For in vitro diagnostic use.
Not available in all countries; please inquire.
FDA listed
References
Ordering Information
To order, please:
Log into your account ► Create a new account ►
For product returns please review our: