Inoculate, Culture, Transport
All in one
With an integrated CO2 system


InTray® GC
Quick Links: Specifications Detection Incubation Storage Whitepapers
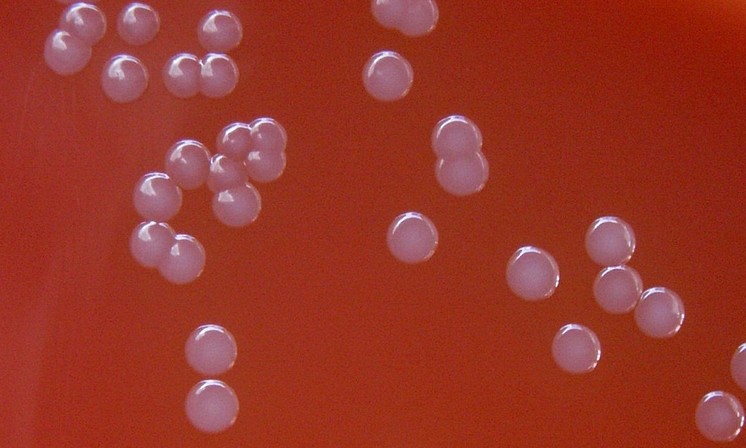
Photo: Positive visual detection of gonorrhea on the InTray GC selective media — within 24-48 hours
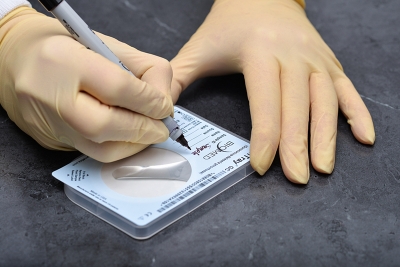
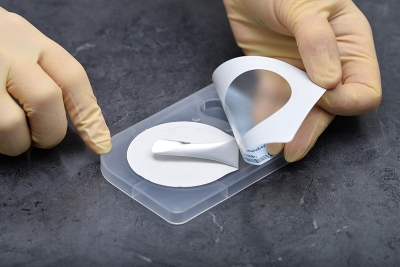
Qualitative detection of oral, rectal and genitourethral Neisseria gonorrhoeae colonization.
Neisseria gonorrhoeae is a common sexually transmitted disease organism broadly disseminated throughout the world. InTray GC is a fully enclosed microbiology cassette, which enables sample collection, transport, culture and identification in a single unit. The proprietary modified Thayer Martin agar is selective for gonococcal bacteria. Results can be interpreted after 24-72 hours incubation. InTray GC is a single exposure culture system with dynamic built-in components and features that are designed for user compatibility and ease of detection.
Specifications
Features and Benefits
Articles and Research Papers
Global Healthcare Detection and Response
Centers for Disease Control and Prevention and National Center for Emerging and Zoonotic Infectious Diseases.
Read ►
CDC Doubles Dose of Ceftriaxone for Gonorrhea
Neisseria gonorrhoeae infections have increased by 63 percent, with growing incidence of antibiotic resistance. Antimicrobial stewardship is the driver of CDC recommendations, highlighting the importance of definitive therapy at the first contact with the patient.
Read ►
The CDC has declared Neisseria Gonorrhoeae infections
to be one of the most important threats of antibiotic resistance in the United States and the world.
Read Paper ►
Recovery of Neisseria gonorrhoeae from 4 commercially available transport systems
John R. Papp
Diagnostic Microbiology and Infectious Disease Volume 86, Issue 2, October 2016, Pages 144-147
Read Paper ►
Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: There is no time like the present
M Bodie
Can Commun Dis Rep. 2019 Feb 7; 45(2-3): 54–62
Read Paper ►
Gonococcal antimicrobial susceptibility surveillance in Europe
Results summary 2017
Diagnostic Microbiology and Infectious Disease Volume 86, Issue 2, October 2016, Pages 144-147
Read Report ►