Decrease Your Risk
with Biomed Diagnostics's HAI Prepared-Culture Media
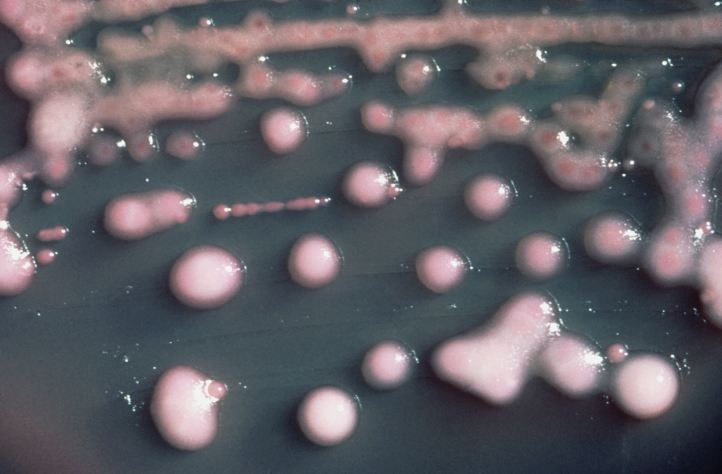
Healthcare Associated Infections (HAI’s), including nosocomial hospital acquired infections
are contagions patients contract while receiving treatment in health care and nursing care facilities.
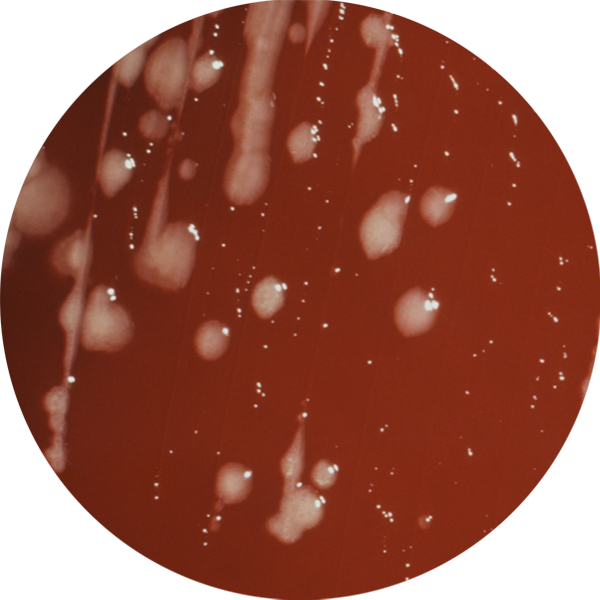
Examples of these serious infections include C. difficile, and detection of multi-resistant bacteria (MRSA, ESBL, VRE). Pre-plated media identification and detection of resistance phenotypes has become critical to the process of microbial genotyping and integration of antimicrobial surveillance stewardship.
Highly Selective and Specific
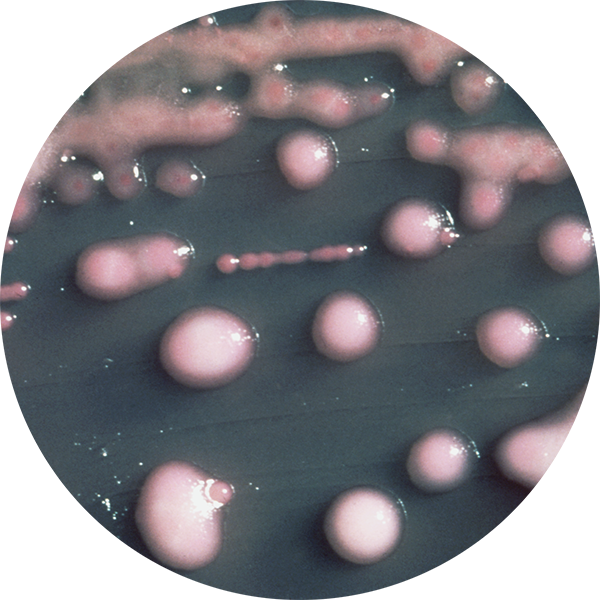
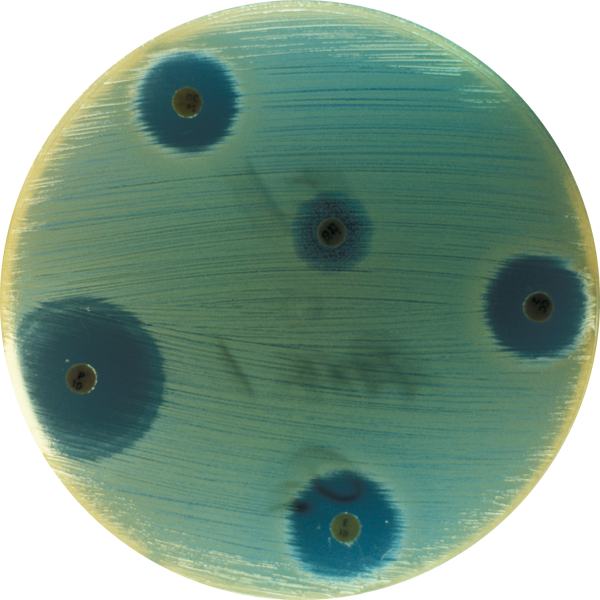
With media that is highly selective and specific to ensure accurate results. Every device offers the convenience of combined sample collection, transport, culturing and result. Due to brilliant, optically clear materials, most Biomed devices can be placed directly on the stage for observation and microscopy. So, no wet mounts are needed – lessening the risk of exposure or contamination.
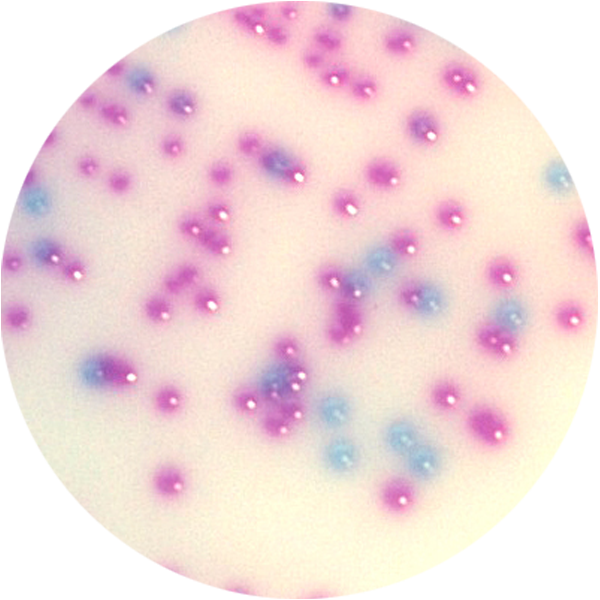
And Biomed’s COLOREX™ series of InTray® devices provides single-step diagnosis based on chromogenic differentiation – usually within 24 hours.
A major, but largely preventable threat
HAI’s present a major, but largely preventable threat to patient and healthcare worker safety. In 2015, the CDC announced a 5-year plan to prevent HAIs. The efforts of these federal multi-agency to prevent HAIs and establish Antibiotic Stewardship (AMR) as well as an HAI Action Plan. This would give facilities at the state, regional and national level the data need to identify problem areas, measure progress of prevention efforts, and ultimately eliminate HAIs.
The Critical Role of Microbiology Labs
The role of microbiology laboratories diagnosing infectious disease has become critical as more antibiotic resistant HAI pathogens emerge. Biomed's prepared-media culture InTray is specifically designed to detect, protect, and lower the risk of harmful HAI contamination bacteria before they infect a patient or staff. Read about Medicare-mandated HAI surveillance reporting and the burden to Infection Prevention stewards.
Prescreening facilities for pathogens as well as testing your equipment and treatment areas for antibiotic-resistant bacteria can be time consuming. However, Biomed’s InTray® devices simplify your workflow -- making it easy to collect and detect resistant microbes — such as Pseudomonas aeruginosa, Methicillin-Resistant Staphylococcus aureus, Acinetobacter, and Burkholderia (Pseudomonas) cepacia.
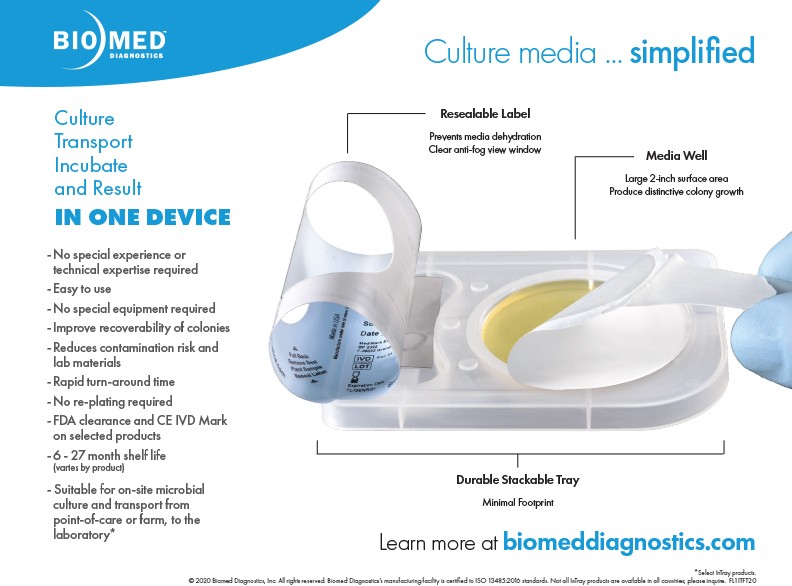
The robust and high-performance, patented design, of Biomed Diagnostics’ InTray in-vitro diagnostic devices help users save time and money, improve workflow / throughput, all while reducing exposure and contamination. By combining multiple procedures: sample inoculation, transport and culture, into a single stackable microbiology cassette, your workflow is streamlined — from point-of-care sample inoculation, all the way to observing InTray directly under the microscope. There's no need to separately prepare a wet mount slide as the fully enclosed system controls air exchange to protect against exposure and contamination. Plus, every InTray has shelf-life of 6 months or more; helping to prevent inventory loss.