Infectious Disease and Culture Diagnostics
As pathogenic organisms increasingly evolve mechanisms to resist current treatment therapies, especially antibiotics. Live culture diagnostic testing is imperative to conduct susceptibility analysis.
Treatment failure is costly and can be deadly
Urinary Tract Infections that are not being treated with an effective antibiotic are landing women in the emergency room and the hospital with severe infections that have spread into their kidneys or become deadly sepsis infections.
Trichomonas vaginalis (TV) is an infection that impacts hundreds of millions of people each year. TV is becoming increasingly resistant to the most prescribed treatment, Metronidazole (MTZ). When the infection persists, the patient can develop acute urogenital inflammatory conditions, infertility, and increased vulnerability to cervical cancer and HIV.
Gonorrhea has declared an urgent public health issue by the CDC due to the resistance of the bacteria to all but one class of antibiotics. There was a 63% rise in GC in the US since 2014. Improperly treated Neisseria gonorrhoeae can have severe reproductive health consequences: infertility, ectopic pregnancy and increase risk of HIV infection. It can rapidly develop antibiotic resistance.
Hospital Acquired Infections manifest significant resistance to antibiotics – and present a major, but largely preventable threat to patient and healthcare workers.
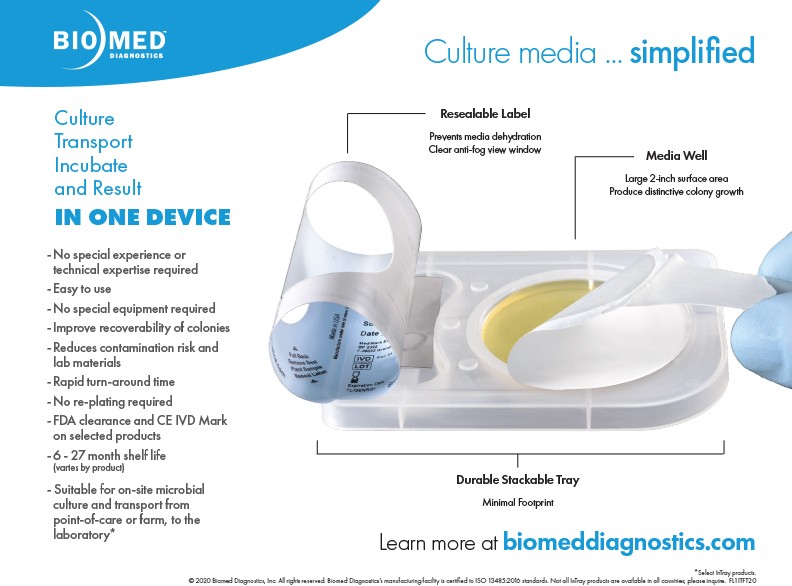
Culture Diagnostics — Made Easy
A single device for sample collection, microbial culture, transport, incubation, and results Simplifies culture media diagnostics and facilitates susceptibility testing.
- For doctors this means a rapid, accurate cost-effective diagnosis so they can prescribe appropriate treatment.
Sending a viable organism safely, from point-of-care to the laboratory, which may take several hours to days, poses several challenges.
- ensuring safe sample collection and transport
- live organism stability until it reaches the lab, for culture of viable isolates
Biomed Diagnostics has engineered a novel culture media device that combines collection, transport, and culture in a single platform. InTray and the InPouch are designed for POC specimen collection and inoculation. The device and selective media enable culture growth from the point of collection which eliminates the need to replate.
Engineered for Live-Organism Transport and Culture — Simultaneously
Designed for ease of use, any staff can be easily trained to inoculate InTray or InPouch with a specimen.
- Bring the device to the patient -- in the field or in the office
- Sample collection, inoculation, transportation, incubation and observation — are combined in one device
- Maintains sample viability from point of collection through result.
- Ensure safety for both the sample and your staff; once inoculated there is no need to reopen — it can be transported, incubated, and then directly placed and viewed under a microscope through the optically clear, antifog window
- Presumptive positive identification typically within 24 hours
- Reduction in time, materials, and loss due to spoilage: 12 – 27 month shelf life, compactness of footprint, no breakage or compromised samples.
- Additional isolates may be harvested from colony growth in the InTray
A Personalized Approach
Biomed takes a personalized approach with our customers — we listen and partner with you to meet the needs of your lab or clinic, and provide in-service product training. InTray devices can be configured with nearly any prepared-media selective for infectious pathogens. Below is a short list of pathogenic organisms for which we’ve created strain-selective tests:
Candida
C. albicans | C. glabrata | C. krusei | C. tropicalis
Citrobacter
C. freundii
Enterobacter
E. cloacae | E. aerogenes
Enterococcus
E. faecalis | E. gallinarum | E.casseliflavus | E. faecium
Escherichia
E. coli
Klebsiella
K. pneumoniae
Listeria
L. ivanovii | L. monocytogenes | L. innocua
Neisseria
N. gonorrhoerae
Pseudomonas
P. aeruginosa
Salmonella
S. enterica | S. typhimurium | S. enteritidis | S. paratyphi A
S. dysgalactiae | S. pyogenes | S. agalactiae | S. pneumoniae
Staphylococcus
S. aureus | S. epidermidis | S. saprophyticus | S. xylosus
Trichomonas
T. vaginalis
Trichophyton
T. mentogrophytes | T. rubrum
Vibrio
V. parahaemolyticus | V. alginolyticus | V. harveyi | V. fischeri | V. vulnificus